standardize sodium thiosulfate
To 500 ml of this solution add 2 g of potassium iodide and 3 ml of 2 M hydrochloric acid and titrate with the sodium thiosulphate solution using the starch solution added towards the end of the titration as an indicator until the blue colour is discharged. Remove air bubbles from the burette and adjust the reading to zero.

Chrominfo Preparation And Standardization Of 0 1 N Sodium Thiosulphate
Sodium thiosulphate is usedin the analysis of iodine.

. The 250 ml primary standard solution is prepared by a precisely weighed amount of the potassium bromate is dissolved in its water. First sodium bicarbonate is added to a iodate-free solution of potassium iodide. Sodium thiosulphate is readily obtainable in a state of high purity but there is always some uncertainty as to the exact water content.
This video represents a complete procedure for the. Titrated with sodium thioslphate. Standardization of sodium thiosulfate using potassium dichromate In this process first we need to liberate iodine to react with sodium thiosulfate.
You can standardize the solution with KMnO4 y acid mediun using also KI. KIO 3 5KI 3H 2 SO 4 3K 2 SO 4. The mixture is shaken until the salts dissolve then conc.
Titrate the sample solution with sodium thiosulphate until the endpoint is reached. Among the variables affecting the stability of thiosulphate solutions are the pH the presence. The concentration of a sodium thiosulfate solution 3 g to 8 g standardized by coulometric titration was for example 208653 mmol kg 1 RSD 0007 n 5 RSD means relative standard deviation and n is the number of measurements under a repeating condition.
01 N sodium thiosulphate Potassium iodate Potassium iodide PRINCIPLE. 005M iodine standardization against thiosulfate This procedure is in fact one of the two based on the reaction of thiosulfate with iodine. If you want to determine the unknwon concentration of a given solution by volumetric titration using another solution whose concentration is known this concentration of this must be standardized to be sure of its exact value.
Take 1000 ml of prepared solution of potassium iodate and pour into an iodine flask. Vikas Jain Lived in Hisar Haryana India Upvoted by Avantika Negi. To standardise 0025 N sodium thiosulfate.
This method is developed using an automated titration system Excellence Titrator. For 10 minutes place the flask in the dark Protect from light. Part I - The Standardization of Thiosulfate Solutions In general thiosulfate solutions are standardized by indirect methods Primary-standard oxidizing agents.
50 ml of distilled water are added to each three of it from graduated cylinder and constantly shake it until the KHP solution are completely Premium Sodium hydroxide Titration. Three sample of 07 09 g of solid KHP are place into each of the three numbered Erlenmeyer flasks. Sodium thiosulfate Sodium thiosulfate sodium thiosulphate is an inorganic compound with the formula Na 2 S 2 O 3x H 2 O.
Sodium Thiosulfate solutions are almost exclusively used to standardize Iodine solutions or as back-titrants in titrations using Iodine. The solutions of sodium tiosulphate Na2S2O3 should be kept in special bottle to Continue Reading Martin J Pitt Former Chartered Chemist and Chartered Chemical Engineer Author has 96K answers and 155M answer views 3 y Related. In the analysis of iodine starch solution is used as indicator.
In this process first we need to liberate iodine to react with sodium thiosulfate. Take 100 mL distilled water in conical flask Add 2g of Potassium di-iodide Add 10 mL of potassium di chromate And lastly add 2 mL of Sulfuric acid Now. Hydrochloric acid is added.
Potassium iodate a strong oxidizing agent is treated with excess potassium iodide in acidic media which liberates iodine. Firstly wash out a burette twice with distilled water. Add 2 drops of starch indicator solution.
Leaving Cert Chemistry- By kind permission of Folens. The principle of sodium thiosulphate standardization is based on redox titration utilizing the iodometric method in which potassium bromate is used as the oxidizing agent. Clean the washed burette with the approximately 01 mol dm-3 standardised sodium thiosulphate which is going to be prepared.
The principle of standardization of sodium thiosulphate is based on redox iodometric titration with potassium iodate as primary standard. The solid is an efflorescent loses water readily crystalline substance that dissolves well in water. The substance is therefore unsuitable as a primary standard.
The mixture is shaken until the salts dissolve then conc. Standardization of sodium thiosulfate using potassium dichromate. Potassium iodate a strong oxidizing agent is treated with excess potassium iodide in acidic media which liberates iodine which is back titrated with sodium thiosulphate Procedure.
The first section is to standardize the Sodium Hydroxide by titration. Why does sodium thiosulphate need to be standardized. Solutions of Sodium Thiosulfate are most commonly standardized with Potassium Dichromate or Potassium Iodate solutions which generate Iodine from Iodide.
Starch indicator is typically used. Typically it is available as the white or colorless pentahydrate Na 2 S 2 O 3 5 H 2 O. Standardisation of Sodium Thiosulphate.
Introduction Sample Preparation and Procedures Chemistry Instruments and Accessories Method in Detail. 1 ml of 01 M sodium thiosulphate is equivalent to 0002784 g of KBrO 3. The principle of standardization of sodium thiosulphate is based on redox iodometric titration with potassium iodate or potassium bromate as a primary standard.
Uniformity of reactions between iodine and sodium thiosulphate forms basis for utilizing the standard solution of iodine in the analysis of sodium thiosulphate. First sodium bicarbonate is added to a iodate-free solution of potassium iodide. Chemical and reagent preparation is very crucial for any test.
We must prepare chemicals and reagents to get the accurate test results. ASTM D1510-16 also describes the standardization method for sodium thiosulfate solution using KIO 3 KI as a primary standard. 2S 2 O 32- I 2 S 4 O 62- 2I - If we have iodine solution of known concentration we can easily use it as a standard for thiosulfate solution standardization and vice versa.
Hydrochloric acid is added.

How To Prepare Standardization 0 1 N Potassium Dichromate Potassium Dichromate Starch Solution Potassium Iodide

0 05 M Edta Solution Preparation Standardization Liquid Hand Soap Liquid Body Wash Diy Hand Soap

Standardization Of Sodium Thiosulphate Solution With Standard

Standard Preparation Chemistry Experiments Life Science Chemical Reactions

How To Prepared 0 1n Sodium Thiosulphate Preparation And Standardise Of 0 1n Sodium Thiosulphate Youtube

Standardisation Of Sodium Thiosulphate 0 02 Molar Solution Of Sodium Thiosulphate Youtube

Preparation And Standardization Of Sodium Thiosulfate By Generose Carumba
Standardize Sodium Thiosulfate According To Astm D1510 16
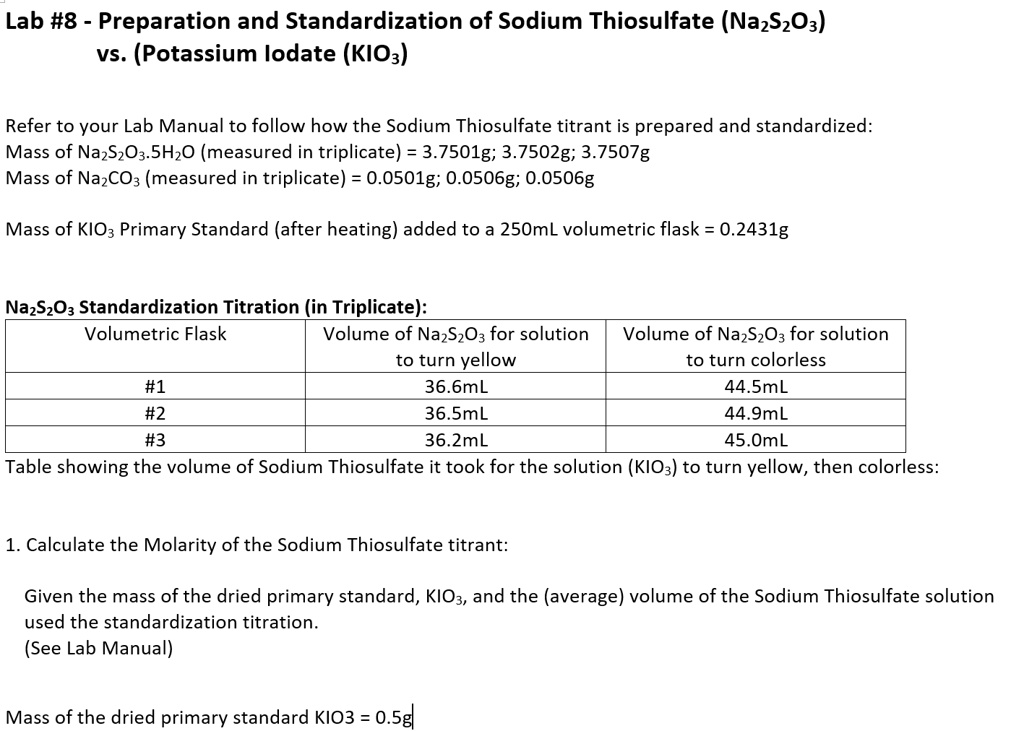
Lab 8 Preparation And Standardization Of Sodium Thios Itprospt
Sodium Thiosulfate 1 0n Standardized Solution Thermo Scientific

Preparation Standardization Of Sodium Thiosulphate 0 1n Sodium Thiosulphate Bp102t L 9 Youtube

0 1 N Silver Nitrate Solution Preparation Standardization

0 1 N Sodium Thiosulfate Preparation And Standardization

How To Prepare Standardization 0 1 N Sodium Hydroxide Naoh Sodium Hydroxide Sodium Preparation

Comments
Post a Comment